gravimetric volatilization method|volatilization gravimeter test : trading In this lesson, we will learn how to use volatilization gravimetry to calculate the quantity of analyte in a sample or determine the formula of a hydrated compound.
eFootball 2024-CPY. In the year 2024, the world of football is about to change forever. Prepare to step onto the virtual pitch like never before in the heart-pounding, adrenaline .
{plog:ftitle_list}
Restaurantes perto de mim. Apetece-lhe algo? Descubra restaurantes perto de si e receba refeições à sua porta. Localizações populares. Lisboa Food Near Me. Porto Food Near Me. Cascais Food Near Me. Sintra .
Unlike precipitation gravimetry, which rarely is used as a standard method of analysis, volatilization gravimetric methods continue to play an important role in chemical analysis. Several important examples are discussed . Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participates in a chemical reaction. In some situations, .
Volatilization methods can be either direct or indirect. Water eliminated in a quantitative manner from many inorganic substances by ignition is an example of a direct determination. It is collected on a solid desiccant and its mass determined by the gain in mass of the desiccant. Another direct volatilization method involves carbonates which generally decompose to release carbon dioxide when acids are used. Because carbon dioxide is easily evolved when heat is ap.
Gravimetry includes all analytical methods in which the analytical signal is a measurement of mass or a change in mass. When you step on a scale after exercising you are making, in a .We learnt that volatilization gravimetry involves separating volatile compounds from a sample and measuring the change in mass. This quantitative analytical method can be used to determine the mass of the remaining nonvolatile substances and, from this, the mass of the . In this lesson, we will learn how to use volatilization gravimetry to calculate the quantity of analyte in a sample or determine the formula of a hydrated compound.
Learn how to determine the purity of a mixture using volatilization gravimetry, and see examples that walk through sample problems step-by-step for you to improve your chemistry knowledge and.
Unlike precipitation gravimetry, which is rarely used as a standard method of analysis, volatilization gravimetric methods continue to play an important role in chemical analysis. Several important examples are discussed below.
Learn about gravimetric analysis, a method to determine the amount of a substance by measuring its mass.Gravimetry or gravimetric analysis is an analytical method used to measure mass or change in mass to quantify an analyte. Putting these two definitions together, we get a new definition.
Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participate in a chemical reaction. In a direct precipitation gravimetric analysis, for example, we convert a soluble analyte .Gravimetric analysis is a quantitative method used in analytical chemistry to determine the amount of a substance present in a sample by measuring its mass. This technique relies on the principles of precipitation and weighing to isolate .This is a summary to accompany "Chapter 8: Gravimetric Methods" from Harvey's "Analytical Chemistry 2.0" Textmap. This is a summary to accompany "Chapter 8: Gravimetric Methods" from Harvey's "Analytical Chemistry 2.0" Textmap. . In volatilization gravimetry, thermal or chemical energy decomposes the sample containing the analyte. The mass of . The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant .
volatilization gravimetry chemistry
Volatilization gravimetry. The analytical technique of gravimetric volatilization separates the masses using thermal or chemical energy to determine their masses. In this method solid reactant molecules are converted into gaseous molecules using thermal or chemical energy. . Electro gravimetric method is employed to separate the ions of a .
volatilization gravimeter thermogram
Method 925.10 in Official Methods of Analysis, 18th Edition (AOAC International, 2007) provides an approved method for determining the moisture content of flour. A preweighed sample is heated for one hour in a 130 o C oven and transferred .If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.What is Gravimetric Analysis? Gravimetric analysis is a method in analytical chemistry to determine the quantity of an analyte based on the mass of a solid. Example: Measuring the solids suspended in the water sample – Once a known volume of water is filtered, the collected solids are weighed. The principle of Gravimetric Analysis: Evaluating Volatilization Gravimetry. The scale of operation, accuracy, and precision of a gravimetric volatilization method is similar to that described in the last section for precipitation gravimetry. The sensitivity of a direct analysis is fixed by the analyte’s chemical form following combustion or volatilization.
Ask the Chatbot a Question Ask the Chatbot a Question gravimetric analysis, a method of quantitative chemical analysis in which the constituent sought is converted into a substance (of known composition) that can be separated from the sample and weighed. The steps commonly followed in gravimetric analysis are (1) preparation of a solution containing a known weight of .
Gravimetric methods: The . quantitative methods. that are based on determining the . mass. of a . . Volatilization gravimetry: The analyte is separated from other constituents of a sample by converting it to a gas of known chemical composition that can be weighed. . A gravimetric precipitating agent should react specifically or at least .Gravimetric analysis is a method in analytical chemistry that uses mass measurement to quantify the quantity of an analyte (the ion being studied). The masses of two substances containing the analyte are compared in gravimetric studies. . Volatilization Method. The process of separating components of the mixture by heating or chemically .
Learn how to determine the purity of a mixture using volatilization gravimetry, and see examples that walk through sample problems step-by-step for you to improve your chemistry knowledge and skills.
Topic Outcomes • Explain and differentiate the theory and practice of precipitate, volatilization and particulate gravimetry • Determine the quantitative, qualitative application of gravimetric method Overview of Gravimetric • Encompasses .Gravimetric Methods Chapter Overview 8A Overview of Gravimetric Methods 8B Precipitation Gravimetry 8C Volatilization Gravimetry 8D Particulate Gravimetry 8E Key Terms 8F Chapter Summary 8G Problems 8H Solutions to Practice Exercises G ravimetry includes all analytical methods in which the analytical signal is a measurement of mass or a change .A gravimetric method in which the . signal is the mass of an electrodeposit . on the cathode or anode in an electrochemical cell. Aqueous ion: determination of Pb. 2+ by oxidizes to PbO. 2. and deposited on Pt anode (chemical converting) Volatilization gravimetry. A gravimetric method in which the . loss of a volatile species . gives rise to
Method 925.10 in Official Methods of Analysis, 18th Edition (AOAC International, 2007) provides an approved method for determining the moisture content of flour. A preweighed sample is heated for one hour in a 130 o C oven and transferred to a desiccator while it cools to room temperature. Four primary gravimetric analysis methods are used to determine an analyte's mass. Outside of precipitation gravimetry, other common methods include volatilization gravimetry, electrogravimetry .
cold compression test outboard
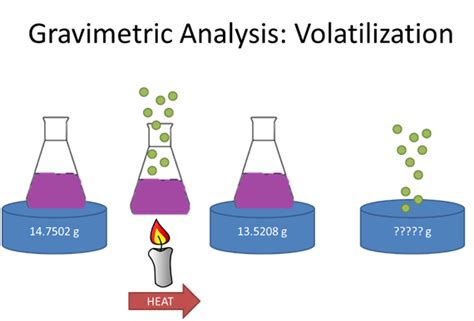
Gravimetric Methods Chapter Overview . 8C Volatilization Gravimetry 8D Particulate Gravimetry 8E Key Terms 8F Chapter Summary 8G Problems 8H Solutions to Practice Exercises G ravimetry includes all analytical methods in which the analytical signal is a measurement
cold compression test results
Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participates in a chemical reaction. In a direct precipitation gravimetric analysis, for example, we convert a soluble analyte into an insoluble form that precipitates from solution. In some situations, however, the analyte . Gravimetric analysis is a quantitative method for accurately determining the amount of a substance by selective precipitation of the substance from an aqueous solution. The precipitate is separated from the remaining aqueous solution by filtration and is then weighed. Assuming that the chemical formula for the precipitate is known and that the .
The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant . Method 925.10 in Official Methods of Analysis, 18th Edition (AOAC International, 2007) provides an approved method for determining the moisture content of flour. A preweighed sample is heated for one hour in a 130 o C oven and transferred to a desiccator while it cools to room temperature.
The gravimetric factor is a conversion factor used in gravimetric analysis to relate the mass of a precipitate formed during a reaction to the amount of analyte present in the original sample. This factor is crucial for accurately determining concentrations in methods that rely on mass measurements, such as precipitation and volatilization, which are common approaches in .Volatilization method • In this method the analyte or its decomposition products are volatilised (dried) and then collected and weighed. • or alternatively, the mass of the volatilise product is . Gravimetric analysis avoids problems with temperature fluctuations, calibration errors, and other problems associated with . Key Terms: Buchner Funnel, Filtration, Gravimetric Analysis, Mass, Titration, Volatilization, Volumetric Analysis. What is Gravimetric Analysis. Gravimetric analysis is a process of measuring the amount of an analyte by its mass. Hence, it .
volatilization gravimeter test
WEBWatch Jenn Todryk, a social media creator and host of No Demo Reno, transform homes without major demolition in the Dallas-Fort Worth area. Learn about her design .
gravimetric volatilization method|volatilization gravimeter test